What is the battery?
The answer to this question is as simple as a device to store energy. Batteries do not provide energy. They are just electronic devices that store the energy and flow it to the circuit when it’s needed. The mechanism to store or release the energy of the battery is involved with the chemicals in the battery since a small change in the mentioned chemicals will affect the storage or release of the energy. Rechargeable batteries repeat the process of chemicals to charge or discharge the battery. Since batteries are not 100% efficient some energy loss occurs due to the charge/discharge process and it’s different for various types of batteries and can differ from 5%-25%.
Figure 1-what is a battery?
Different principles affect batteries. One of them is the temperature. The reason behind the increase in battery temperature is internal resistance. Internal resistance accounts for energy.
The performance of a battery is another principle. The performance of a battery actually depends on how the batteries are used. Various types of batteries represent different performances in electronic circuits. Lead-acid batteries, for example, demonstrate 85-95% efficiency while alkaline and NiCad batteries are about 65% efficient. Some deep cycle AGM’s (Absorbed Glass Mat) can reach 98% efficiency under optimum conditions but it occurs rarely, so normally 10-20% energy loss in battery setups is expected. Simply, a battery is represented as figure 1. Different types of batteries and their characteristics will be discussed in this article.
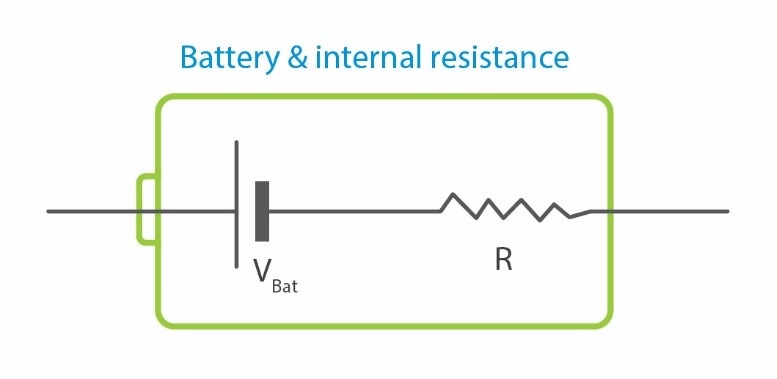
Figure 2- topology of a practical battery
How batteries are distinguished:
There are several characteristics associated with a battery among which capacity and depth of discharge are used to rank a battery.
Voltage: The voltage of a battery is a fundamental characteristic that is determined by its chemicals. Different batteries come in different voltages such as 2V, 3.7V, 6V, and 12Volts.
Capacity: The charge stored in the battery is known as the capacity of the battery. The maximum amount of energy provided by a battery on a specific condition is called capacity. However, the nominal capacity of the battery can change noticeably in different conditions. Various factors including depth of charge/discharge, age, and climate conditions can affect the nominal capacity of the battery.
Depth of Discharge: While the battery is employed in a system, an amount of stored energy will be withdrawn which is measured by the depth of discharge (DOD). Many types of batteries are not fully discharged unless they are seriously damaged. For example, if the DOD of a battery is given by the manufacturer as 30%, then only 30% of the battery capacity can be used by the load.
Batteries have an international technical standard which is called C charging ratio and this standard is demonstrated through 0.1C, 0.2C, C20 and so on. This means that for example if the battery is 100 AH, in 1C condition, the charging or discharging current is 100A. Also, for C20, C20 stands for 0.05C charging ratio, so a 100AH battery will be empty after 20 hours in C20 standard. Figure 3 illustrates the discharge characteristics of a sample battery for different C charging ratios.
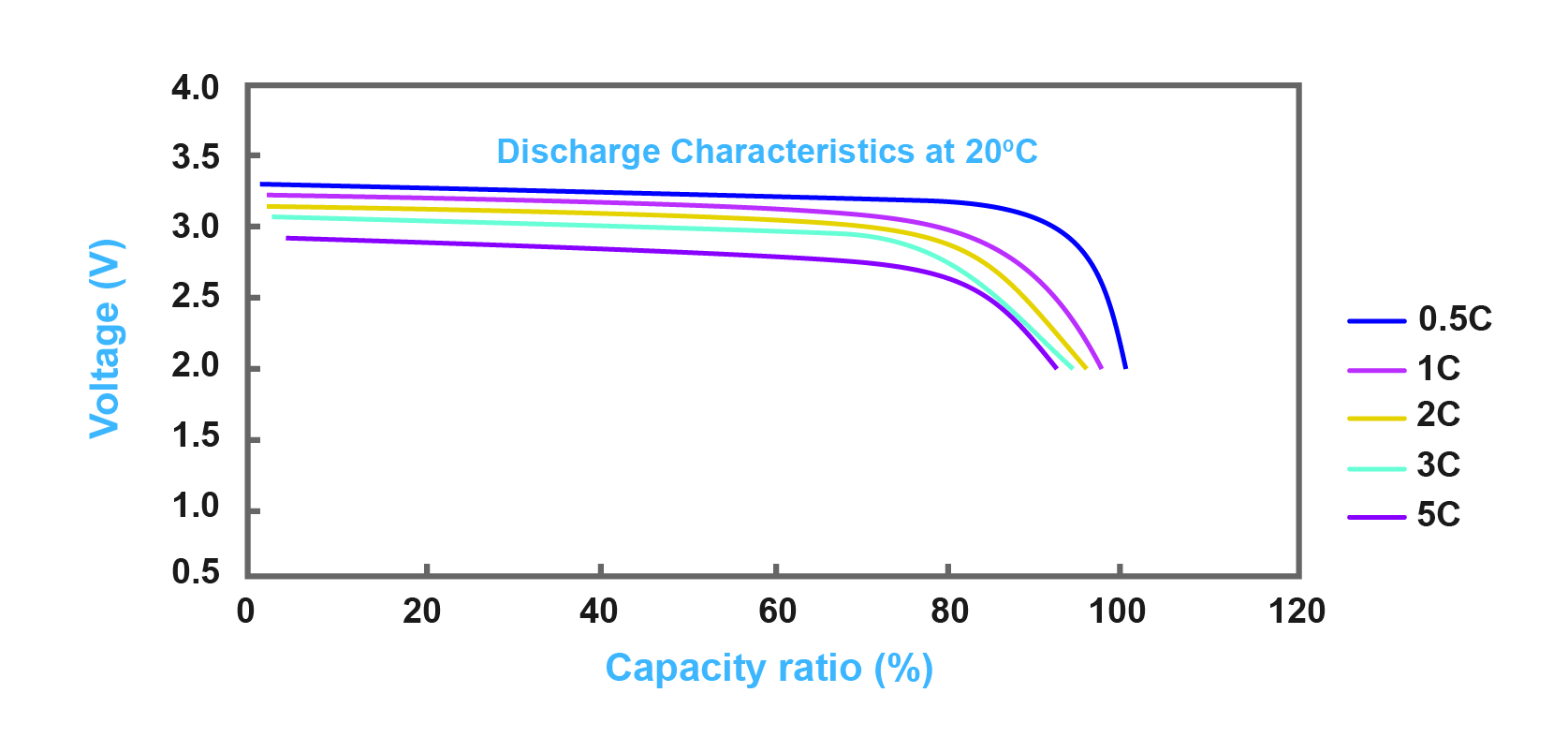
Figure 3- discharge characteristics of lithium battery with different C charging ratios
Types of Batteries
Various types of batteries are applied to different applications. Batteries are categorized according to two principles: application and construction. For large systems, common applications are usually automotive, marine and deep-cycle. Photovoltaic systems, backup power, traction and boat batteries are specific areas for deep-cycle batteries. According to construction batteries are classified into flooded, gelled and sealed AGM batteries.
Depending on the energy sector, one might use different kinds of batteries. AGM, VRLA, Lead Acid, and Lithium and etc. might look confusing so in this article, we will try to make it easy to understand the most common types of batteries and the differences between them.
Automotive (Starting) Batteries
This type of batteries are employed to start/run the engines, that’s why they’re also called starting batteries. Engine starters are in need of a very large amount of current for a short time. Therefore lots of thin plates are employed to achieve maximum surface area and as a result higher starting current in starting batteries. While these kinds of batteries perform well as sudden current providers, they are not proper for deep-cycle applications. For a PV application, for example, the plates of automotive batteries which are composed of sponge will be quickly consumed and fall on the bottom of the cell so their lifetime won’t exceed 150 cycles whereas they do demonstrate a long lifetime for regular starting cycles.
Figure 4- Starting battery
Deep-Cycle Batteries
These batteries are composed of thicker plates which are made of solid leads. This will lead to lower surfaces and accordingly less instant power, unlike the starting batteries. They specifically designed for deep cycling applications. Thick plates reduce the total surface area, resulting in a battery that provides lower max current, but at the same time capable of a deeper state of charge. Deep cycle batteries are typically discharged down to 50% of their nominal capacity. This property makes them suitable for applications where a constant current is needed for long periods of time. Deep-cycle batteries can be discharged down to 20% of their nominal capacity. However, it’s recommended not to discharge them deeper than 50% of their nominal capacity. It is also recommended to fully charge those batteries once in a month to maintain the battery’s levels of capacity. Otherwise, the capacity will be reduced over time. Deep Cycle batteries are available in various types such as AGM or GEL batteries.
Figure 5- Deep-cycle battery
Marine Batteries
The characteristics of marine batteries are between starting and deep-cycle batteries and they are also called as “hybrid” batteries. Hybrid batteries are made of coarser and heavier spongy plates. These batteries act interstitially as starting and deep-cycle batteries.
Figure 6- Marine battery
What Is Inside A Battery?
Here the mechanism and construction of a battery will be discussed. Most of the available industrial rechargeable batteries are lead-acid batteries. To understand the internal structure of a battery, we take the lead-acid batteries as the basic example in this article. Several single cells with an approximate voltage of 2.1V are connected in series and construct together with the battery. For example, a six-volt battery has three single cells and a twelve-volt battery has six single cells in series. Each cell consists of two lead plates. One of these two plates is a positive plate which is covered with lead dioxide and a negative plate made of sponge lead. There is also a separator in between. The plates are submerged in an electrolyte consisting of water and sulfuric acid within an enclosure. Each cell is capable of storing 2.1 volts. Figure 7, shows the structure of a single cell.
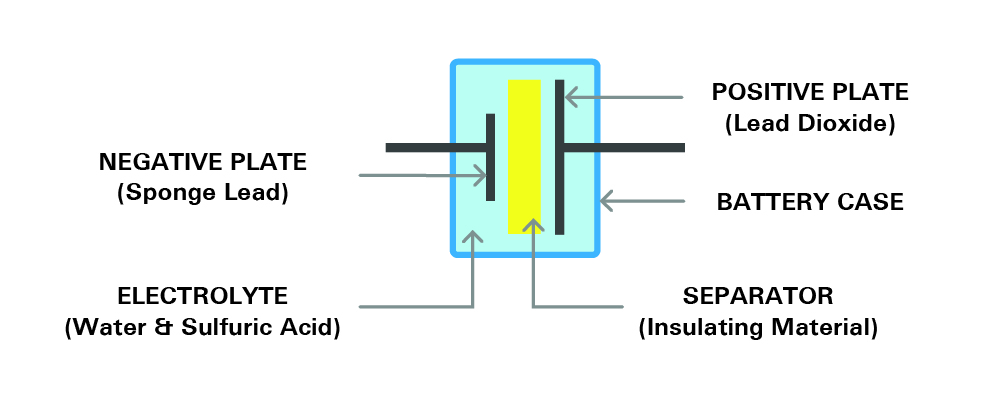
Figure 7- the structure of a cell
Six 2.1-volt cells are connected in series to make the typical 12-volt battery. This battery is shown in figure 8. This 12v battery will produce a total voltage of 12.6V after being fully charged.
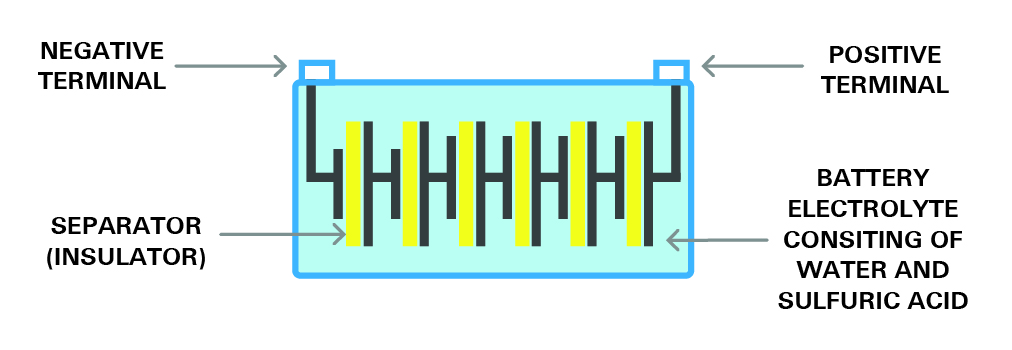
Figure 8- the structure of a cell
The lifetime of a Battery
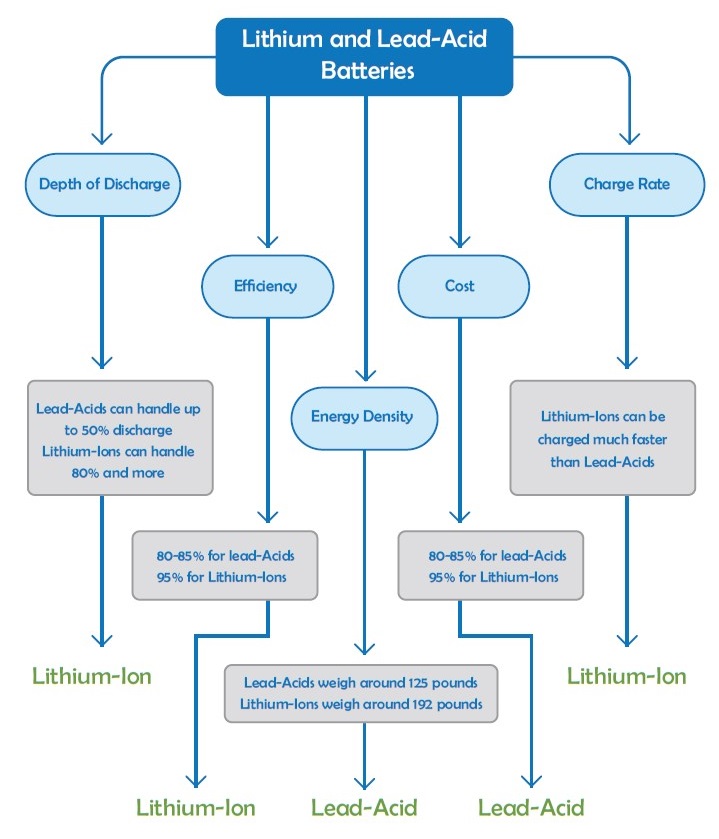
The lifetime of a battery highly depends on how it is used. Maintenance, charging process and climate conditions are also important factors in the life of a battery. A battery can die for severe overcharge in less than a year, while suitable care and maintenance can drive a battery under heavy service over long years. Usually, lead-acid batteries are prone to danger due to the higher rates of discharge. If a lead-acid battery is discharged deeply (over 80%) it loses its full capacity in next charge state, this is while lithium-ion batteries do not have a charging memory. This means that they can be discharged deeply without being destroyed.
Temperature is another principle in determining a batteries life. Batteries function best at room temperature. Operating a battery at higher temperatures improves performance but prolonged exposure will shorten life. All batteries achieve optimum lifetime if used at 20°C. In increased temperatures, the cycle life is reduced by 20%, 30%, 50% and even higher.
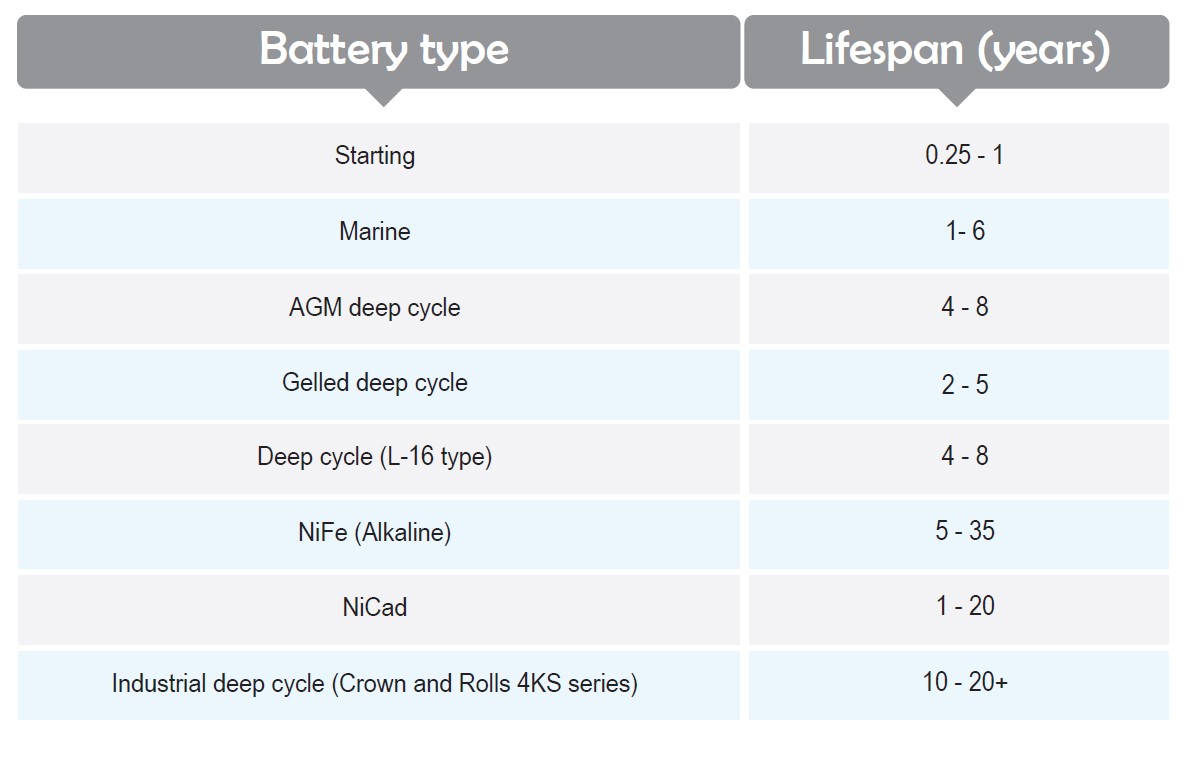
Here are typical expectations of various types of batteries in normal maintenance conditions:
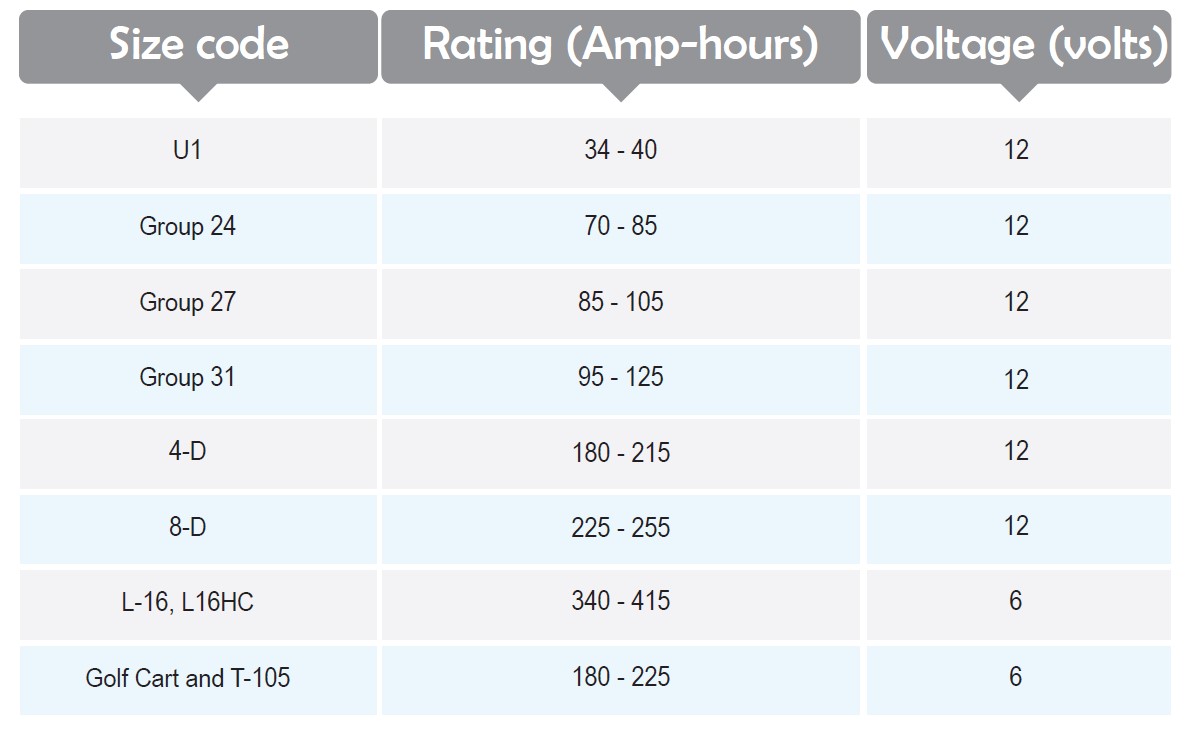
Battery Size Codes
Batteries are different in size so that a standard code system is developed to categorize them. However, many batteries do not belong to any of these codes, but it is a nice reference to adjust the physical size and terminal placement of the batteries. The rating of the batteries is illustrated in Amp-hours which stands for the current during an hour, for example, if one uses a 60 amps battery in 15 minutes time cycles the rate would be 60×0.25=15 amp-hours.
Photovoltaic Batteries
An important part of every off-grid solar system is the battery. The energy demand for energy rises in the evening, this is while solar energy is mainly generated during the day. Using a battery, solar power produced during the day would be stored and used when it is actually needed.
In this section, the batteries of solar systems and the advantages or disadvantages of various types will be discussed in detail. This section will lead to a proper choice for different solar setups. Solar batteries are commonly included in the deep-cycle category which provides energy for renewable energy systems. Deep-cycle batteries are involved with repeated and deep discharge processes which make them different from other battery types such as automobile batteries.
Four main types of batteries are commonly employed in solar systems and other renewable energy systems. In this article, these types will be discussed in detail.
1) Lead-Acid
Sealed lead-acid batteries are a group of this family that is maintenance-free. Several types of sealed lead acid have emerged and the most common are GEl, also known as valve-regulated lead-acid (VRLA), and absorbent glass mat (AGM). Electrodes of this type are grids of metallic lead-containing lead oxides which change during the charge/discharge process. They are bulky and occupy more space than other types of batteries, so they can’t be considered a suitable choice for “on-grid” applications. Before recent achievements, lead-acid batteries were the only practical choice to store solar energy. The combination of AGM technology and lead-acid batteries make them as one of the best batteries to employ in solar systems. AGM batteries are not gelled electrolytes and are considered as “recombinant gas absorbed electrolyte” which cuts water loss up to 98%. AGM batteries have the lowest amount of internal resistance compared with other batteries and their self-discharge loss is 3-10 times less than gelled batteries and 5-50 times less than old flooded batteries. AGM sealed battery technology was invented in 1980 and developed for military aircraft in 1985 to test the reliability of these kinds of batteries.
Lead-acid batteries benefit from a long life which is highly dependent on the type of battery for example industrial batteries can live up to 20 years while standard ones live for 3-5 years. It should be noted that this lifespan is a matter of careful maintenance. This type of batteries also suffers from practical downsides. Figure 8 demonstrates the capacity of lead-acid batteries after deep cycles. It can be noted that the battery decreases to 20% capacity for 3800 cycles.
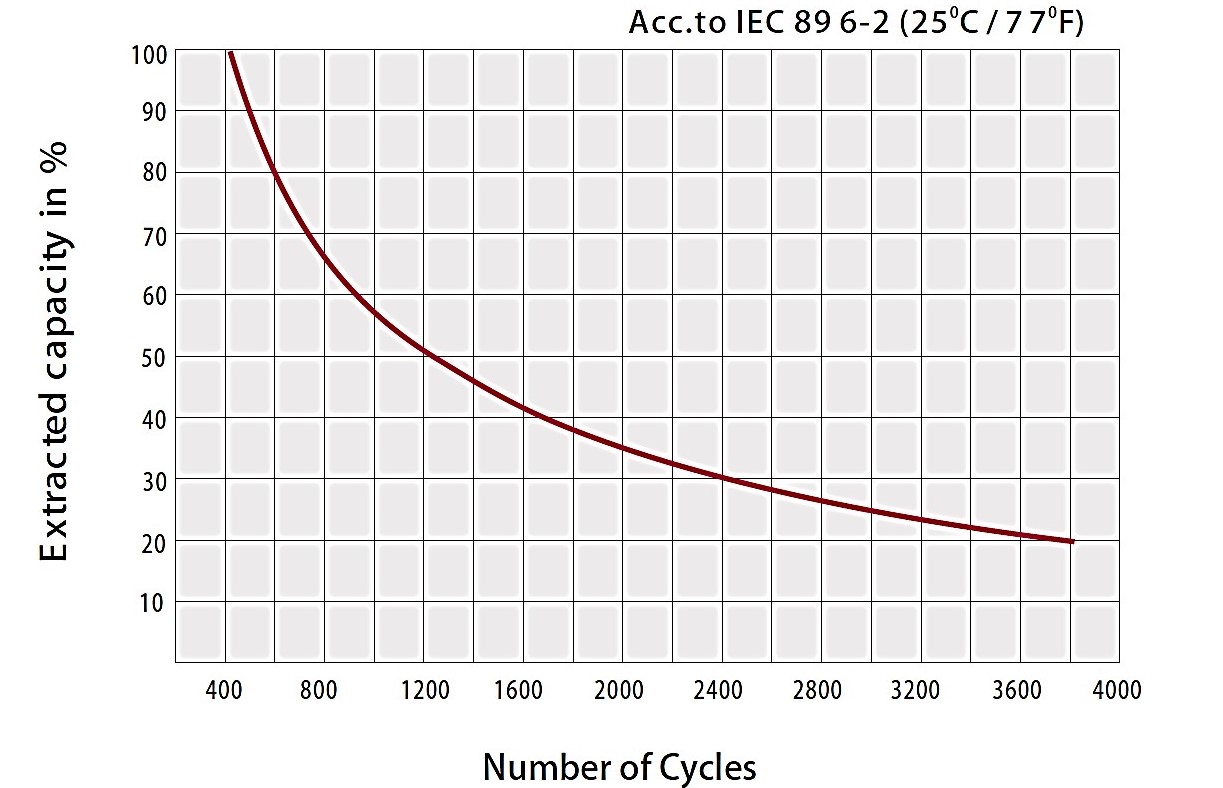
Figure 9-lifespan of a lead-acid battery
2) Lithium-Ion Batteries
Lithium batteries have many advantages over traditional battery types including long life cycle and higher charge/discharge rates. The chemical used in lithium batteries which are employed in solar systems is Lithium Iron Phosphate (LiFePO4) which demonstrates great thermal stability, high current ratings, and long life cycle. Unlike flooded lead-acid and others battery chemistries lithium batteries do not vent dangerous gasses such as hydrogen or oxygen so they are less involved with the risk of explosion. Each cell of lithium battery has the nominal voltage of 3.6V while lead-acid battery cell can provide 2V so for a constant voltage lithium battery needs fewer cells than lead-acid battery but they have very similar charging voltages that can be easily replaced with each other. Lithium batteries do not need to be fully charged regularly, while lead-acid batteries suffer from plate degradation when their discharge process starts before they get fully charged. Another important factor is performance efficiency where lead-acid batteries represent 80% efficiency during their life cycle, while this amount can reach 95-98% by lithium-ion batteries. The main downside for the lithium batteries is their higher price compared to lead-acid batteries at the moment.
3) NICAD (Nickel Cadmium)
In this type of batteries, the positive electrode is made of nickel oxide, and the negative one contains cadmium. Alkaline batteries are suitable for emergency or standby systems, but not for daily cycles, so they are not recommended for solar systems. NICADs are very expensive, and cadmium is hazardous material for human beings and also they suffer from low efficiency (65-80%).
Some other types of solar batteries such as Sodium Nickel Chloride, NIFE (Nickel-Iron) and flow are also employed in solar systems while their life-cycle (about 3000-4000 cycles) and higher expenses limit their application since lead-acid and lithium batteries are economic choices compared to the alkaline or flow batteries. Besides the low life-cycle, low efficiency is another disadvantage of alkaline batteries.
Lead-Acid and Lithium Batteries
In this section, Lead-acid and Lithium batteries will be discussed in detail since they are the best choices to employ in solar and backup systems. Their life-cycle, cost, and efficiency make them proper to these kinds of applications. Here various types of lead-acid batteries will be introduced. The cells of lead-acid batteries are generally 2V, so for 12V, we need six cells in series.
Flooded Lead-Acid Batteries
This type is widely employed in automotive industries and can be considered as the most economical choice for solar systems. Normal flooded batteries require extra care and regular maintenance for proper watering, equalizing charges and keep the terminals clean. Flooded batteries should be transported carefully and they have specific transportation rules.
Sealed Lead-Acid Batteries
The thickness of the battery plates determines the ability and application of the battery. SLAs do not tend to be sulfate or as wet cell batteries, so they’re the safest type of lead-acid batteries. SLAs are categorized to AGM and Gel Cells. They are packaged in a plastic container and mainly used in small UPS, emergency lighting and wheelchairs. The low price, reliability and low maintenance are the prominent features of these batteries. They are recommended in hospitals, retirement homes, internet hubs, banks and etc. their performance is bad at low-temperature climate conditions and it gets worse for below 0℃ temperatures.
AGM Sealed Lead-Acid Battery
Absorbed Glass Matt (AGM) process is prior to traditional flooded technology. These types of batteries could be charged faster than other types of batteries due to the lower internal resistance. AGM batteries are able to provide higher capacity while their size is smaller than other types and they are shipped by regular and standard shipping regimes. The AGM suspend the electrolyte in a designed glass-mat which leads in the faster-charging process and instant high load currents, so they are suitable for starters of motorcycles, hybrid-cars, and large solar systems.
With cycling, the capacity of AGM decreases gradually; gel, on the other hand, demonstrates high performance for a longer time but involves a sudden performance decrease about the end of its life. AGM batteries cost less than gel batteries while they are more expensive than flooded ones. Gas generating is prevented in sealed lead-acid batteries by voltage control. Water depletion, dry-out, and gassing are results of overcharging the battery. VRLA (voltage regulated lead-acid) battery is optimized to perform at 25℃; the battery’s lifespan cuts to half by every 8℃ increment.
Gel Sealed Lead Acid
Gel VRLA batteries contain a gelled electrolyte separates them from AGM counterparts. Sulfuric acid is mixed with silica fume makes the resulting mass gel-like which makes them maintenance-free. This type can be shipped through normal methods and does not require any additional cares. GEL batteries are persistent to higher temperatures, shock, and vibration. Over discharging causes serious damages to Flooded and some AGM batteries while gel batteries are not involved with the overcharging/discharging issue. They are the proper choice for constant current applications. Gel batteries perform well at low-temperature conditions which make them prior to sealed batteries.
Gel batteries are more expensive than AGM ones and they have a very low discharge rate (approximately 1% per month) but they are associated with their specific gel chargers and should be accompanied by them.
Lithium Batteries
Lithium-ion batteries are becoming increasingly accepted over lead-acid batteries because of their various advantages. Lithium-ion batteries are now considered as the standard in PV systems. According to the lifetime, lithium-ion batteries are expected to last around 15 years which is about five to ten years higher than lead-acid batteries. On the other hand, ventilation is not necessary for lithium batteries. And the most important advantage of the lithium-ion batteries is that they discharge up to 100%. However, the deeper discharge will reduce their lifespan. Lithium batteries have two important features which the battery disconnects the input and output of the battery when the voltage of battery exceeds the maximum protection voltage and the same procedure was applied to the output of the battery when the output voltage is too low. The second important feature is about temperature conditions where for overheating battery the input and outputs are disconnected and for temperatures below 0℃ the input of the battery and for below than -20℃ the output is disconnected in lithium batteries.
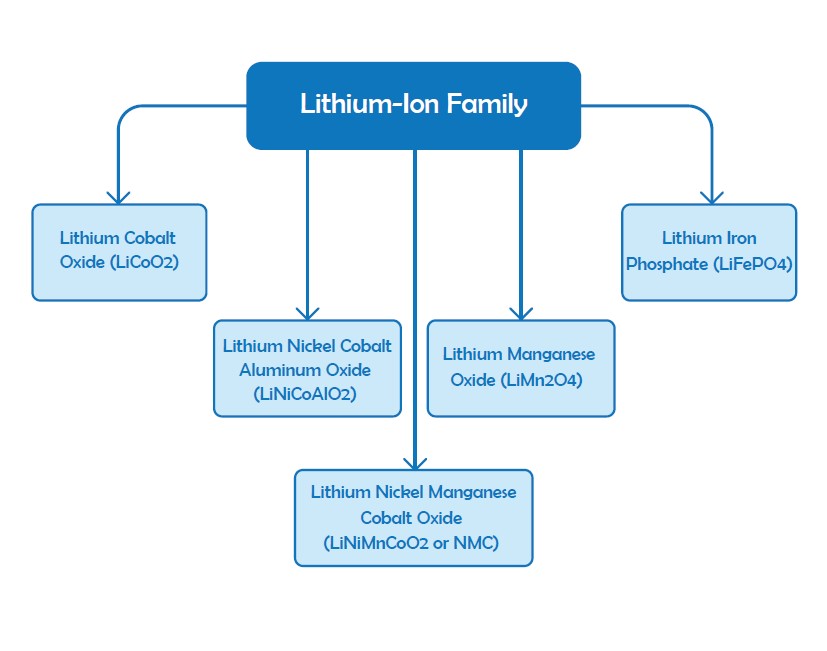
The term “lithium-ion” stands for a family of batteries with noticeable similarities but different chemistries such as Li-cobalt, LI-Manganese, NMC and Li-Aluminum, Li-Phosphate, Li-Titanate, and Lithium Iron Phosphate. As mentioned in the previous sections Lithium Iron Phosphate (LiFePO4) is of the common choices for solar applications because of the long lifetime, deeper cycles, maintenance-free and no venting characteristics. Li-Manganese, NMC and Li-Aluminum and Li-Phosphate are employed in portable applications because of their higher capacity. Li-Titanate and Lithium Iron Phosphate have lower voltages and less capacity, but are very enduring. The setup of lithium batteries will be expensive than lead-acids but their efficiency and long lifespan can be a good reason to utilize them in PV systems. Lithium batteries provide 3.6V voltage for each cell which makes them as a proper choice for mobile devices to reduce the cost of multi-cell designs. These types of batteries require regular protection and they are among the expensive batteries.
EPEVER has introduced its latest product line: Lithium batteries, enhancing our comprehensive solar energy solutions for our valued customers.